Belotero ®
Product Summary
Launched in the UK in January 2007, Belotero® is a new monophasic double crosslinked hyaluronic acid created in Switzerland by Anteis, and distributed in the UK by Merz Pharma UK Ltd.
Generic name
Non-Animal Hyaluronic Acid (NaHA) gel.
What does it contain?
Belotero® has been designed from biofermentation hyaluronic acid, a substance naturally present in our body; which the makers claim makes it safe and completely biocompatible, as well as wholly biodegradable.
How is it made?
It is created using Cohesive Polydensified Matrix (CPM) technology, a unique process which gives Belotero® hylauronic acid unusual elasticity; (this technolology adds to its “mono-phasic” nature).
Belotero® therefore guarantees a considerably better result from the first hours after the injection and over the initial months, in terms of increased volume and wrinkle filling compared to “biphasic” products.
Belotero® is prepared from non-animal, hyaluronic acid NaHA by a patented dynamic reticulation process. The unique polydensified cohesive structure of Belotero® consists of a cohesive gel which is easy to inject through a small diameter needle.
Belotero® is prepared from non-animal, hyaluronic acid NaHA by a patented dynamic reticulation process. The unique polydensified cohesive structure of Belotero® consists of a cohesive gel which is easy to inject through a small diameter needle.
Is a skin test required before treatment?
No allergy test needed.
Is it temporary or permanent?
Hyaluronic acid is completely broken down within the skin over a period of months, eventually leaving no trace of the filler.
Licenced status
Medical device.
Should be used by
Trained members of the medical profession only.
Product range
Belotero® has three main different formulations within it`s range: Basic, Soft and Intense.
Belotero® Basic (22.5mg/ml of NaHA) is an ideal product for volume enhancement treatments and can be used for deep furrows, lip augmentation, facial outlines, naso-labial furrows, glabellar lines and the correction of facial depressions, either due to injury or age-related scaring. It is injected into the medium to deep dermis with a 27G needle.
Belotero® Soft (20mg/ml of NaHA) is an ideal substance for superficial wrinkles and can be used for perioral wrinkles, lip commissures, crow`s feet, and forehead wrinkles. It is injected into the upper dermis with a 30G needle.
Belotero® Soft (20mg/ml of NaHA) is an ideal substance for superficial wrinkles and can be used for perioral wrinkles, lip commissures, crow`s feet, and forehead wrinkles. It is injected into the upper dermis with a 30G needle.
Belotero Intense (25.5mg/ml of NaHA) is an ideal product for deeper lines and wrinkles. It is injected into the deep dermis with a 27G needle.
Not to be used in
Individuals with a known hypersensitivity to hyaluronic acid.
Duration of effect
This depends upon the degree of correction required, your age and lifestyle as well as the correct placement of the product by a practitioner.
The manufacturers claim that Belotero® is long lasting and effective from 6 to 9+ months and suggest that two treatments per year are sufficient for an elegant, natural result.
The manufacturers claim that Belotero® is long lasting and effective from 6 to 9+ months and suggest that two treatments per year are sufficient for an elegant, natural result.
Reported side effects
Transient erythema (redness), swelling, pain, itching, discoloration or tenderness at the implant site. Typically resolution is spontaneous, within one or two days after injection into the skin, and within a week after injection into the lips.
The manufacturers state that there is no risk of granulomas and no risk of ‘lump effect’ – although the product may be too new to identify these rare reactions which can occur with other hyaluronic acid fillers.
The manufacturers state that there is no risk of granulomas and no risk of ‘lump effect’ – although the product may be too new to identify these rare reactions which can occur with other hyaluronic acid fillers.
Costs
This depends on the area treated and how much is required, and the practitioner doing the treatment, but price ranges are in the region of £300 per treatment.
Clinical results
Fuctionality Study: Dr Bezzola - Switzerland, Dr Luiggi - France, Dr Micheels - Switzerland, Dr Reynaud - France.
Tissue augmentation by injection of Belotero®
The objective of this investigation is to confirm the safety and performance of Belotero® device for face tissue augmentation. This study, realized by 4 very skilled investigators, on 31 patients between 26 and 80 years old, in different injection sites highlights:
Tissue augmentation by injection of Belotero®
The objective of this investigation is to confirm the safety and performance of Belotero® device for face tissue augmentation. This study, realized by 4 very skilled investigators, on 31 patients between 26 and 80 years old, in different injection sites highlights:
- The safety of the product: no immediate, short or mid term side effects have been mentioned, except some redness that disappear few hours after the treatment.
- The optimized ease of use of the product , thanks to the ergonomy of the syringe and the behavior of the product under the skin, comparing to competitive products.
- The very nice aesthetic result that Belotero® procures immediately and within the first month.
Clinical results of male nasolabial lines treated with Belotero®.
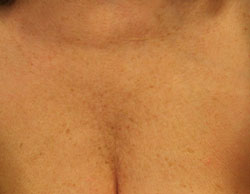
Female, décolletage (area between next and breasts) before treatment. | Female, décolletage 6 weeks post treatment with Belotero®. |
Images above provided courtesy of Dr.Geoff Fairris, Dermatologist at the Wessex Skin Clinic.
Female, nasolabial (nose to mouth) lines before treatment. | Female, nasolabial immediately post treatment with Belotero®. |
Images above provided courtesy of BotoMedics Ltd.
Before and after photographs are real patients, your results may differ.
After having proved the safety of the product for the patient during this functionality study, Anteis has planned to complete the first results concerning short term performance and to launch a long term evaluation of the product.
Update: 13/12/11 - This filler is now FDA approved





People tired to find a relevant place where they can know real facts and myths about the topic mentioned by author of this blog. I want to help a lot of needy people through this blog to come up at my blog to know the real facts and myths regarding this topic.
ReplyDeleteโบท๊อก หน้าเรียว
THANKS FOR SHARING THIS INFORMATION ABOUT THIS BLOG. Belotero Filler Treatment in Islamabad,
ReplyDeleteThanks for sharing beneficial information and also visit my blog for better understanding Is Belotero a good filler in Dubai
ReplyDelete